Here at Rady Children’s Institute for Genomic Medicine, we are always looking for ways to make whole genome sequencing (WGS) available to more critically ill patients. That’s why we’re excited to announce our newest partnership with Inozyme Pharma to expand access to WGS for infants suspected of having Generalized Arterial Calcification of Infancy (GACI).
Inozyme is seeking patients with disease-causing variants in the ENPP1 and/or ABCC6 genes for possible participation in a clinical trial. To help find those patients, Inozyme has offered to pay for ultra-rapid whole genome sequencing in critically ill babies who meet specific criteria.
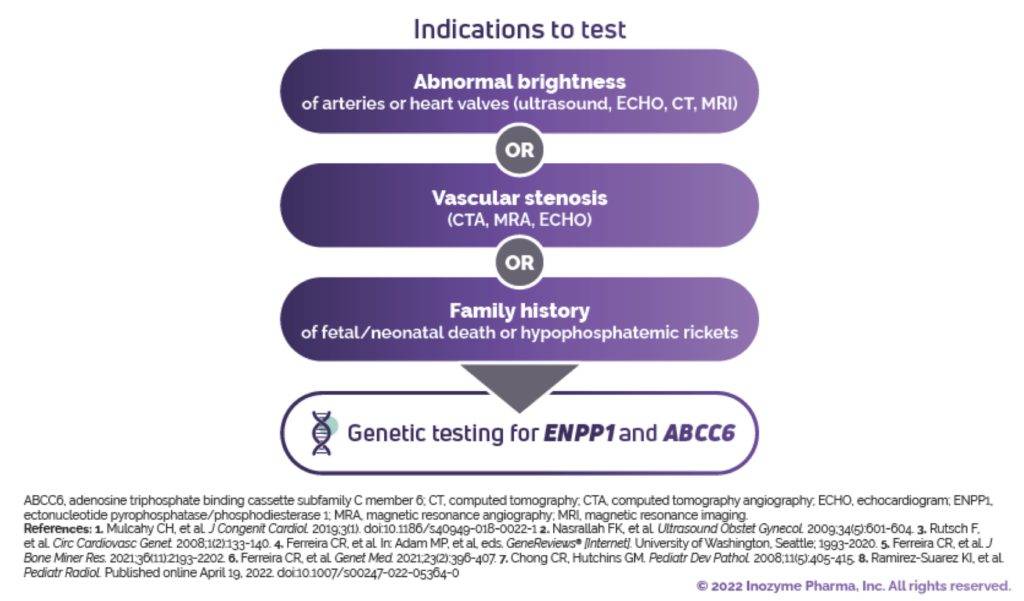
Indications to test include:
For More Information:
If you have a patient who you think qualifies for this sponsored testing, please contact Catherine Nester at Inozyme as soon as possible. We anticipate this opportunity to be available for the next 6-12 months, so even if you do not have a patient who meets criteria today, please keep this flyer available to refer to if a patient presents at a later date.
- To learn more about ENPP1 Deficiency, visit the Inozyme website.
- Rare Community Profiles: Inozyme’s Catherine Nester Discusses Newborn Screening and the GACI Diagnostic Delay