— Jason Knight, MD
Medical Director- PICU
CHOC Children’s Hospital of Orange County




Our genomic experts include research scientists, as well as board-certified medical geneticists, genetic counselors, neonatologists, perinatologists, and pediatric subspecialists spanning various fields.


— Jason Knight, MD
Medical Director- PICU
CHOC Children’s Hospital of Orange County


— Jennifer Burton, MS, CGS
Clinical Genetic Counselor
Children’s Hospital of Illinois
A precise rapid diagnosis can have a profound impact on patient outcomes.
The earlier rapid Whole Genome Sequencing™ is employed, the greater the potential benefit.
When a child is struggling to survive, our goal is to deliver rapid, robust phenotype-driven results in time to make a difference.
We offer clinically evaluated genome-wide testing with demonstrated utility for acute patient indications.
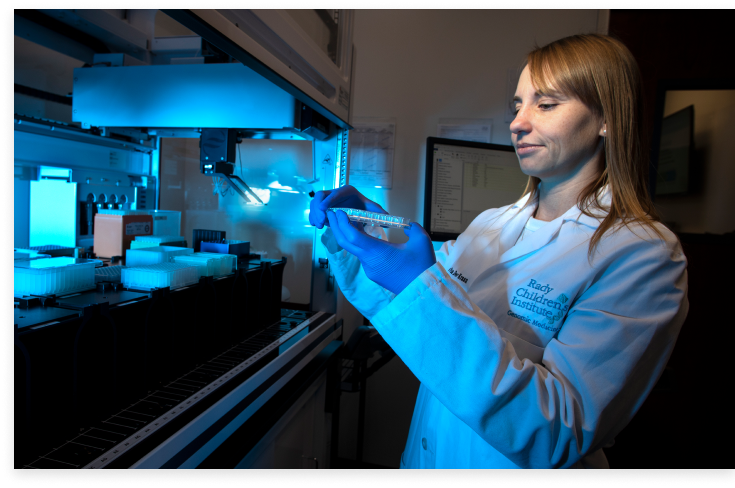
Along with a detailed molecular diagnostic clinical report, our lab and medical directors can support ordering providers by addressing questions related to testing or results in a timely manner.
The entire RCIGM team is driven by a passion for optimizing rapid genomic testing to help medical teams at the bedside provide personalized life-changing treatment for the youngest, sickest patients.
Our Clinical Genome Center is CAP accredited (#9487427) and CLIA certified (#05D12129627). We are licensed to provide clinical diagnostic testing in all 50 states.
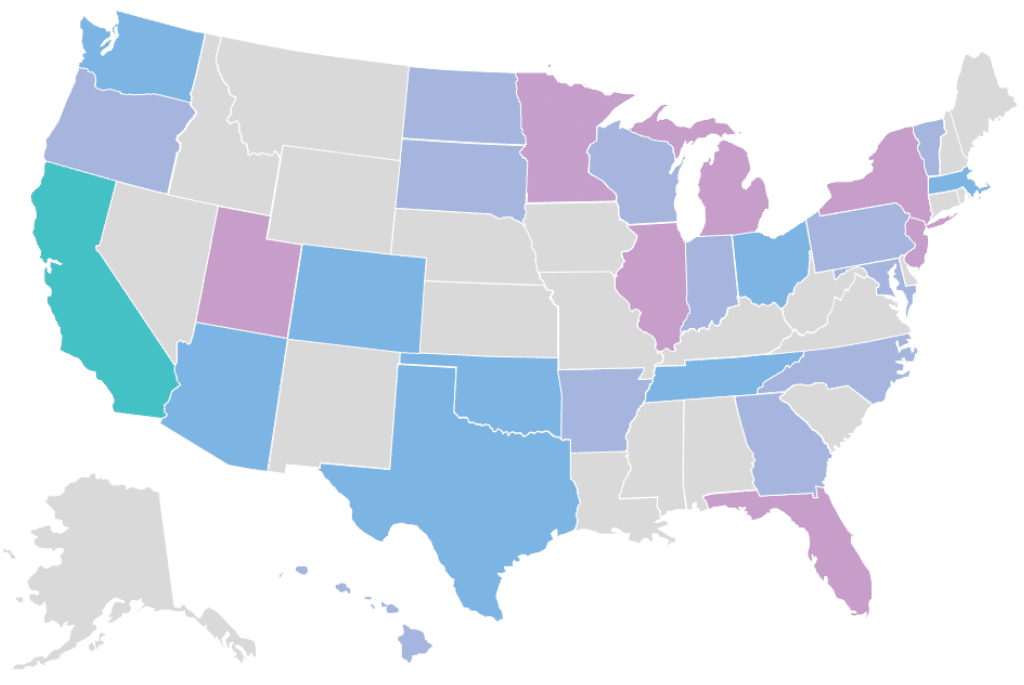
RCIGM also works with healthcare, medical research and life science alliances globally to expand access to whole genome sequencing for genetic disease. Learn more about our collaborations with organizations including the Vermont Oxford Network; the Sanford Children’s Genomic Medicine Consortium; and the Medical Genome Initiative.
rWGS® is best considered for critically-ill infants and children in the inpatient setting for which a rapid genetic diagnosis could impact medical management and outcomes.
Indications for rWGS® testing include:
Benign and Likely Benign variants are not reported. In addition, RCIGM-CGC does not offer reporting on carrier status, pharmacogenetic markers, polygenic risk scores, or genome wide association studies (GWAS) risk variants.
Whether you have questions or are ready to get started, our team is here to help.
© 2025 Rady Children's Institute for Genomic Medicine.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Etiam dignissim posuere semper. Nunc et dapibus turpis, vitae pellentesque nisl.