From 13 Years to 13 Hours: Rady Children’s Institute for Genomic Medicine Demonstrates Fastest Time to Life-Changing Diagnosis for Infants with Rare Disease
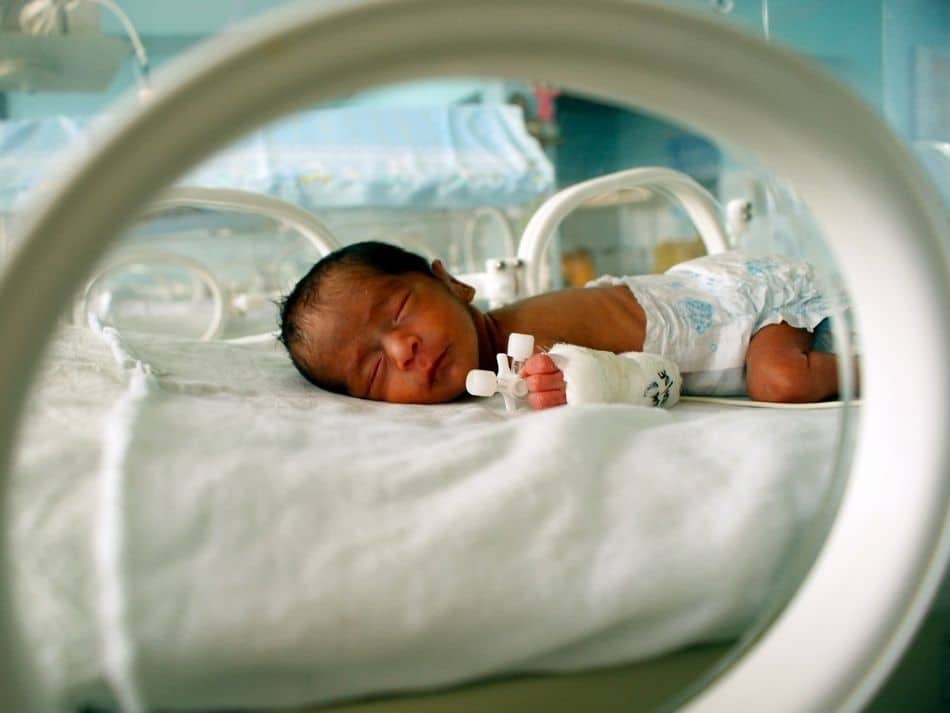
It took an international research effort 13 years to complete the first sequence of the human genome – the code of life. Now, Rady Children’s Institute for Genomic Medicine (RCIGM), in collaboration with Illumina, Inc. and Alexion Pharmaceuticals, Inc., is refining an ultra-rapid sequencing process to diagnose rare disease in 13.5 hours.
Whole-genome-sequencing: navigating the ‘diagnostic odyssey’ in rare disease research

TALKING TECHNIQUES PODCAST: This episode of the Talking Techniques podcast explores the impact of whole-genome sequencing on rare disease diagnosis and treatment, from research to clinical outcomes. Features Dr. David Dimmock from RCIGM.
Opinion: Genome sequencing can lead to life-changing care for infants. California should make it more available

Innovative medical technology is constantly being developed, and with it comes the possibility for incredible breakthroughs in modern medicine. California has never seen scientific advancements within closer reach than they are today. For critically ill infants hospitalized with unexplained rare diseases, the opportunity to benefit from a medical miracle has arrived.
Rapid Genome Sequencing Can Save Babies With Rare Diseases, If They Can Get It

Undiagnosed genetic diseases take a serious physical and emotional toll on families. Rapid genome sequencing can provide answers and guide treatment decisions, but so far, insurance companies have been reluctant to pay. That’s beginning to change.
AdventHealth diagnoses severely underweight baby with rare allergy using genomic testing

A Central Florida family is thanking AdventHealth and genomic testing for a life-saving diagnosis that saved their baby boy. Six months ago, when Michael Ferrara-Urban was three-months-old, he was admitted to AdventHealth for Children (AHFC) for “failure to thrive.” Last April, AHFC partnered with Rady Children’s Institute for Genomic Medicine in San Diego to bring […]
Rady Children’s Institute for Genomic Medicine Receives New York State Approval for Clinical Sequencing

San Diego – February 18, 2021 – Rady Children’s Institute for Genomic Medicine (RCIGM) is now licensed by New York State to perform clinical molecular testing making the Institute one of the few laboratories nationwide approved to perform whole exome and whole genome sequencing (WGS) on samples sent from all 50 states and the District of Columbia.
